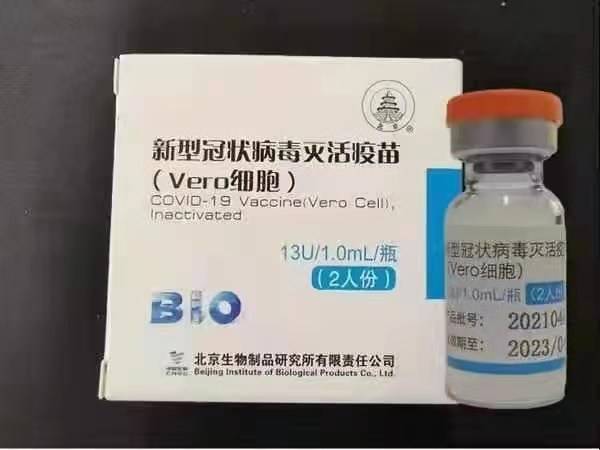
Sinopharm (Beijing): BBIBP-CorV
CHIKAMU 1
1 Muedzo
ChiCTR2000032459
China
CHIKAMU 2
2 Miedzo
NCT04962906
Ajendina
ChiCTR2000032459
China
CHIKAMU 3
6 Miedzo
NCT04984408
ChiCTR2000034780
Mubatanidzwa wenyika dzeArab Emirates
NCT04612972
Peru
NCT04510207
Bahrain, Egypt, Jorodhani, United Arab Emirates
NCT04560881, BIBP2020003AR
Ajendina
NCT04917523
Mubatanidzwa wenyika dzeArab Emirates
Mvumo
WHO Emergency Use Listing 59 Nyika
Angola 、 Argentina 、 Bahrain 、 Bangladesh 、 Belarus 、 Belize 、 Bolivia (Plurinational State of) 、 Brazil 、 Brunei Darussalam 、 Cambodia 、 Cameroon 、 Chad 、 China 、 Comoros 、 Egypt 、 Equatorial Guinea 、 Gabon 、 Gambia 、 Georgia 、 Guyana 、 Hungary 、ndonesia 、 Iran (Islamic Republic of) 、 Iraq 、 Jorodhani 、 Kyrgyzstan 、 Lao People's Democratic Republic
Lebanon 、 Malaysia 、 Maldives 、 Mauritania ur auritius 、 Mongolia 、 Montenegro 、 Morocco 、 Mozambique 、 Namibia 、 Nepal 、 Niger 、 North Macedonia 、 Pakistan 、 Paraguay 、 Peru 、 Philippines 、 Republic of the Congo 、 enegal 、 Serbia 、 Seychelles 、 Sierra Leone 、 Solomon 、 Islands 、 Somalia 、 Sri Lanka 、 Thailand 、 Trinidad and Tobago 、 Tunisia 、 United Arab Emirates 、 Venezuela (Bolivarian Republic of) 、 Viet Nam 、 Zimbabwe
Sinopharm BBIBP-CorV COVID-19 mushonga usina kugadzirwa wakagadzirwa kubva mutsika-yakakura hutachiona hutete husina hutachiona hwehutachiona. Mukwikwidzi wekudzivirira uyu akagadzirwa neSinopharm Holdings neBeijing Institute of Biological Products.
Mushonga weSinopharm BBIBP-CorV unoshanda nekubvumira immune system kuti ibudise masoja anorwisa SARS-CoV-2 beta coronavirus. Mishonga yekudzivirira hutachiona yakashandiswa kwemakumi emakore, senge jekiseni rerabi uye chirwere chehepatitis A. Iyi tekinoroji yekusimudzira yakashandiswa zvinobudirira kumajekiseni mazhinji anozivikanwa, senge jekiseni rerabhi.
Sinopharm's SARS-CoV-2 strain (WIV04 strain uye raibhurari nhamba MN996528) yaive yakasarudzika kubva kumurwere kuJinyintan Hospital muWuhan, China. Hutachiona hwakaziviswa mutsika mune anokwanisa Vero cell mutsara, uye supernatant yemasero ane hutachiona haana kugadziriswa ne β-propiolactone (1: 4000 vol / vol, 2 kusvika 8 ° C) kwemaawa makumi mana nemasere. Mushure mekujekeswa kwemarara esero uye ultrafiltration, yechipiri β-propiolactone inactivation yakaitwa pasi pemamiriro akafanana sekutanga kusaita. Sekureva kweWHO, jekiseni iri rakasimudzirwa pa06 mg yealum uye yakaiswa mumajekiseni akafemerwa mu0.5 mL yesaline yakasviba-yakasungwa saline isina zvinodzivirira.
Musi waZvita 31, 2020, iyo State Drug Administration yakazivisa kubvumidzwa kwechirwere chekuyedza chakagadzirwa naSinopharm.
Musi waMay 7, 2021, World Health Organisation yakazivisa kubvumidzwa kwechirwere. Rondedzero yeUN yechimbichimbi yekushandisa yakabvumira nyika kuti dzikurumidze mvumo dzadzo dzega dzekupinza nekupa mushonga weECOVID-19. Boka reNyanzvi reWHO rezveMhando Yekudzivirira zvirwere rakapedzisa ongororo yaro. Kubva pane humbowo hwese huripo, WHO inokurudzira mishonga miviri yekudzivirira, mavhiki matatu kusvika mana akaparadzana, kune vakuru vane makore gumi nemasere zvichikwira. Mushonga wekubatsira kurwisa chirwere uye zviratidzo muzvipatara zvinofungidzirwa pa79% kumazera ese akasanganiswa.
IAmerican Medical Association yakaburitsa "A Randomized Clinical Trial: Mhedzisiro ye2 Inactivated SARS-CoV-2 Vaccines paSymbomatic COVID-19 Infection muVakuru" musi waMay 26, 2021, ichipedzisa kuti "mune ino yakatarwa kuongororwa kwenguva pfupi kweyakaitwa kiriniki kuongororwa, vanhu vakuru Iwo maviri akavharirwa maSVS-CoV-2 majekiseni akaiswa mune ino yakatarwa ongororo yepakati yemiedzo yemakiriniki yakasarudzika yakanyanya kudzora njodzi yeCOPVID-19 yezviratidzo, uye zviitiko zvakakomba zvakashata zvaive zvisingawanzoitika. " Muchikamu chino 3 kuyedzwa kusarongeka muvanhu vakuru, kushanda kweiyo 2 isina kugadzikana hutachiona hwehutachiona muzviratidzo zveCOVID-19 zviitiko zvaive 72.8% uye 78.1%, zvichiteerana. Majekiseni e2 aive nezviitiko zvakakomba zvisingaite zvine frequency yakafanana kune alum-chete boka rekutonga, uye mazhinji aive asina hukama nekubaiwa. Ongororo yekuongorora yakawana kuti majekiseni maviri aya akaita kuti zviyero zvakaenzana zvisaenzanisike, zvakafanana nezvakabuda muchikamu che2 / 2 kuyedzwa.
WHO SAGE Working Group yakaburitsa ongororo yemushonga weSinopharm / BBIBP COVID-19 musi waMay 10, 2021. Mushonga weGAVI-COVID-19 unosanganisira vaccine vial Monitor iyo inotaurira vashandi vehutano kana mushonga uyu wakachengetedzwa zvakanaka uye usati waratidzwa. kupisa. Nekuda kweizvozvo, kukuvara, GAVI yakashuma musi waMay 14, 2021. smart labels inogadzirwa neZebra Technologies uye inogadzirwa neTemptime Corporation, ine denderedzwa ine yakareruka kara square mukati, yakagadzirwa nekemikari isina ruvara iyo isingadzoreke inogadzira ruvara nekufamba kwenguva. . Izvi zvinova zvinosviba kupa chiratidzo chekuona chekuwedzera kwekushisa kupisa. Kana bhodhoro iri rangoonekwa nekupisa kupfuura rakaringana nzvimbo yekuchengetera, iyo mraba inove yakasviba kupfuura iyo denderedzwa, zvichiratidza kuti mushonga haufanire kushandiswa.
National Drug BBIBP-CorV COVID-19 yekudzivirira mushonga raibhurari nhamba yekunyoresa: DB15807.